- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
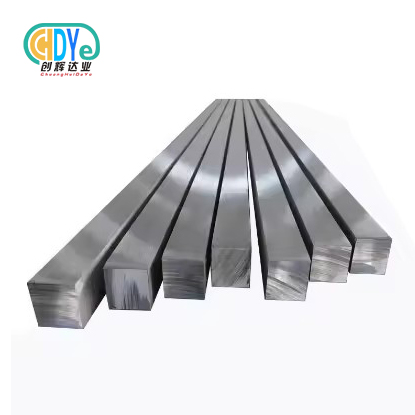
Medical-Grade Titanium Square Bar – Biocompatible Options
Modern medical device manufacture relies on medical-grade titanium square bars for their biocompatibility and mechanical qualities. These precision-engineered materials are essential to surgical instruments, orthopedic implants, and specialized medical equipment that protects patients. The Titanium Square Bar configuration improves structural integrity while being lightweight for medical applications. Understanding medical-grade titanium's requirements and benefits helps procurement professionals make decisions that affect patient outcomes and device performance.
Understanding Medical-Grade Titanium Square Bars
Material Composition and Grades
Multiple alloy compositions of medical-grade titanium square bars are designed for specialized medical applications. Commercially pure grade 2 titanium is good for dental implants and basic surgical tools due to its corrosion resistance and biocompatibility. Grade 5 titanium alloy (Ti-6Al-4V) meets load-bearing orthopedic implant biocompatibility criteria while increasing strength.
Grade 23 titanium (Ti-6Al-4V ELI - Extra Low Interstitial) is the best medical grade due to its reduced oxygen and iron content, which improves ductility and fracture toughness. This grade fulfills ASTM F136 and ISO 5832-3 criteria for maximum performance in sensitive medical situations.
Geometric Properties and Specifications
Square bars are more stable than round bars, resisting torsional pressures and maximizing material efficiency in machining. Standard dimensions are 8x8mm to 200x200mm, with unique specifications for special design needs. Controlled forging and heat treatment ensure homogenous grain structure and mechanical qualities across the material cross-section.
Dimensional tolerances are usually ASTM B348, with precision tolerances for important applications. Bright-polished mirror surfaces for cosmetic medical equipment and machined finishes for processing optimization are available.
Biocompatibility Characteristics
Medical-grade titanium integrates with human flesh without causing immunological responses, making it biocompatible. The passive oxide layer limits ion release that could irritate tissue or cause systemic toxicity. Titanium square bars are ideal for permanent implant applications that require long-term tissue compatibility.
Advantages of Using Medical-Grade Titanium Square Bars
Advantages make medical-grade titanium square bars ideal for demanding medical applications. Beyond biocompatibility, mechanical, chemical, and processing properties improve device performance and production efficiency.
Superior Biocompatibility and Safety Profile
Medical-grade titanium's stable oxide layers prevent hazardous ions from entering tissues, making it biocompatible. Clinical investigations suggest that titanium implants have little inflammatory response and optimum tissue integration over time. This is especially useful in orthopedic applications where implants must last decades.
Titanium implants are non-magnetic, thus patients with titanium implants can undergo diagnostic imaging without safety issues or image abnormalities. This avoids implant removal or replacement during advanced medical imaging.
Outstanding Corrosion Resistance
In the harsh environment of human physiology, medical-grade titanium resists corrosion. The material withstands medical fluids, drugs, and sterilization. Corrosion resistance extends device lifespan and reduces material degradation that could threaten patient safety.
It resists steam autoclaving, gamma radiation, and chemical sterilants. This resilience allows medical equipment to retain their structural and surface qualities after numerous sterilization cycles, allowing surgical instrument reuse.
Optimal Strength-to-Weight Ratio
Medical device design benefits from titanium alloys' high strength-to-weight ratio. Grade 5 titanium has tensile strength similar to many steel alloys but weighs 45% less. This allows the development of lighter surgical instruments that reduce physician fatigue during long procedures while keeping structural strength for accurate medical treatments.
Implants with reduced density minimize patient weight while providing mechanical support for bone replacement and strengthening. Older patients and those with low bone density who need orthopedic surgery benefit from the less weight.
Comparing Titanium Square Bars with Alternative Materials
Titanium versus Stainless Steel
Surgical equipment and temporary implants have typically been made of medical-grade stainless steel. The biocompatibility and corrosion resistance of titanium square bars are significant. Stainless steel contains nickel and chromium, which may induce allergic reactions in sensitive persons, while titanium is biocompatible and has little tissue reactions.
Titanium's corrosion resistance shines in long-term implant applications. In the physiological environment, crevice corrosion and pitting may degrade and liberate ions from stainless steel implants. Titanium doesn't lose its passive oxide layer, therefore implants operate well for life.
Performance Comparison with Aluminum Alloys
Aluminum alloys are lightweight but not biocompatible for medical use. Aluminum ions can accumulate in biological tissues, making it unsuitable for implanted devices due to toxicity. Titanium is preferred for medical applications that require lightweight qualities due to its safety and weight advantages.
Titanium alloys outperform aluminum in high-stress applications. In cyclically loaded medical equipment like bone plates and spinal rods, Grade 5 titanium has better fatigue resistance than most aluminum alloys.
Cost-Effectiveness Analysis
Titanium materials cost more than stainless steel or aluminum, but their total cost of ownership is generally lower in medical applications. The prolonged service life, decreased maintenance, and elimination of material failure replacement operations give considerable economic benefits over the device lifecycle.
Compared to work-hardened stainless steel, annealed titanium has better machinability and lower manufacturing costs. Standard machining can accomplish tight tolerances and complex geometries, reducing extra processing and expenses.
Procurement Insights: Buying Medical-Grade Titanium Square Bars
Quality Standards and Certifications
Understanding standards and certification requirements is essential for buying medical-grade titanium square bars. ASTM F136 specifies titanium alloys for medical uses, while ASTM F67 governs surgical implant unalloyed titanium. International standards for titanium alloy medical devices, ISO 5832-3, ensure universal adoption.
Material test certificates (MTC) should be required for chemical composition, mechanical characteristics, and biocompatibility testing in procurement specifications. Ultrasonic inspection certifications check internal structure and find performance-compromising faults. Dimensional inspection reports verify tolerances and geometry.
Supply Chain Considerations
Medical-grade supplies must meet strict quality and regulatory standards, making supply chain management essential. Medical equipment suppliers must use ISO 13485 quality management systems. Manufacturing, from raw material management to final inspection, meets medical device industry requirements with this accreditation.
Medical-grade titanium has longer lead times than industrial titanium due to quality monitoring and certification. Critical medical device manufacturing processes benefit from early procurement planning to reduce production delays and maintain inventory levels.
Supplier Evaluation Criteria
Suppliers should be assessed for technical skills, quality, and regulatory compliance. Certifications and client references from medical device manufacturers are required for suppliers to produce medical-grade materials. Complete traceability documentation is needed for regulatory submissions and quality audits.
Vacuum arc remelting (VAR) should be used in manufacturing to achieve chemical uniformity and eliminate contamination. Inspection must include destructive and non-destructive examination of material qualities and internal soundness.
Company Introduction and Product & Service Information
Shaanxi Chuanghui Daye Metal Material Co., Ltd. is a leading medical-grade titanium square bar manufacturer with over 30 years of rare metal processing experience. As China's "Titanium Capital," Baoji High-tech Development Zone provides our organization with unmatched raw resources and medical-grade manufacturing infrastructure.
Our extensive product line includes commercially pure titanium (Gr1, Gr2, Gr4) and medical-grade titanium alloys (Gr5, Gr9, Gr23). Each titanium square bar is tested for chemical composition, mechanical properties, and ultrasonic examination to meet international medical device standards.
Advanced Manufacturing Capabilities
Our modern manufacturing facility has vacuum melting furnaces, precision forging equipment, and CNC machining centers to oversee the entire manufacturing process from raw material to completed product. Continuous quality and medical device traceability are achieved with the integrated approach.
Thermal control systems monitor heat treatment stages to optimize microstructure and mechanical qualities. Before medical device manufacturing, skilled metallurgical technicians establish grain structures and ensure all materials fulfill performance standards.
Quality Assurance and Certification
Our ISO 9001:2015 accreditation proves our quality management competence and facilitates medical-grade material manufacture. Comprehensive documentation, statistical process control, and continual improvement ensure product quality and regulatory compliance in our quality system.
Every shipment includes material test certifications with chemical composition, mechanical property, and biocompatibility data. Ultrasonic inspection certificates verify internal integrity and prevent medical device performance issues.
Custom Fabrication Services
Custom fabrication for medical devices is available in addition to titanium square bar items. Our technical team works with customers to optimize material specifications for performance and manufacturing efficiency.
Surface treatments include bright polishing for aesthetics, machined finishes for precision components, and biocompatible or processing-friendly treatments. Custom dimensional tolerances address crucial design needs when standard specs fail.
Conclusion
Modern medical device manufacture uses biocompatible, corrosion-resistant, and mechanically superior titanium square bars. These items are appropriate for surgical instruments, orthopedic implants, and specialized medical equipment that prioritize patient safety because to their outstanding material qualities and geometric stability. To ensure regulatory compliance and device performance, procurement must focus on quality, supplier qualifications, and certification. Product development and supply chain management in this crucial application sector depend on working with experienced manufacturers who understand medical device industry needs.
FAQ
Q: What grades of titanium are suitable for medical applications?
A: Grades 2, 5, and 23 represent the primary titanium alloys approved for medical device manufacturing. Grade 2 provides excellent biocompatibility for basic applications, while Grade 5 offers enhanced strength for load-bearing implants. Grade 23 features the highest purity levels and optimal mechanical properties for critical medical devices.
Q: How is biocompatibility verified for medical-grade titanium?
A: Biocompatibility verification follows ISO 10993 standards through comprehensive testing that includes cytotoxicity, sensitization, and implantation studies. These tests evaluate material interactions with biological systems and confirm safety for intended medical applications. Certification documentation provides evidence of successful biocompatibility testing.
Q: What custom dimensions are available for titanium square bars?
A: Custom dimensions accommodate virtually any medical device design requirement, with cross-sections ranging from small precision components to large structural elements. Length specifications can extend up to 6000mm, with longer dimensions available through special arrangements. Tolerance specifications can be tailored to match precision requirements of specific applications.
Q: What are typical lead times for medical-grade titanium orders?
A: Standard grades typically require 1-2 weeks for delivery, while custom specifications may extend lead times to 2-3 weeks depending on specific requirements. Expedited processing is available for urgent medical device production needs, though additional quality control measures may influence delivery schedules.
Contact Chuanghui Daye for Premium Titanium Square Bar Solutions
Shaanxi Chuanghui Daye delivers exceptional medical-grade titanium square bar products that meet the most demanding biocompatibility and performance requirements. Our three decades of specialized experience in rare metal processing, combined with ISO 9001:2015 certification, ensures reliable quality and complete regulatory compliance for your medical device manufacturing needs. Whether you require standard grades or custom specifications, our team provides comprehensive technical support and competitive factory-direct pricing that strengthens your supply chain effectiveness. Contact our titanium square bar supplier team at info@chdymetal.com to discuss your specific requirements and discover how our expertise can enhance your medical device development projects.
References
1. American Society for Testing and Materials. "Standard Specification for Unalloyed Titanium, for Surgical Implant Applications." ASTM F67-13, 2018.
2. International Organization for Standardization. "Implants for Surgery - Metallic Materials - Part 3: Wrought Titanium 6-Aluminum 4-Vanadium Alloy." ISO 5832-3:2016.
3. Niinomi, M., and M. Nakai. "Titanium-Based Biomaterials for Preventing Stress Shielding Between Implant Devices and Bone." International Journal of Biomaterials, vol. 2011, article ID 836587.
4. Ryan, G., A. Pandit, and D.P. Apatsidis. "Fabrication Methods of Porous Metals for Use in Orthopaedic Applications." Biomaterials 27.13 (2006): 2651-2670.
5. Williams, D.F. "On the Mechanisms of Biocompatibility." Biomaterials 29.20 (2008): 2941-2953.
6. Geetha, M., A.K. Singh, R. Asokamani, and A.K. Gogia. "Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants – A Review." Progress in Materials Science 54.3 (2009): 397-425.
Learn about our latest products and discounts through SMS or email