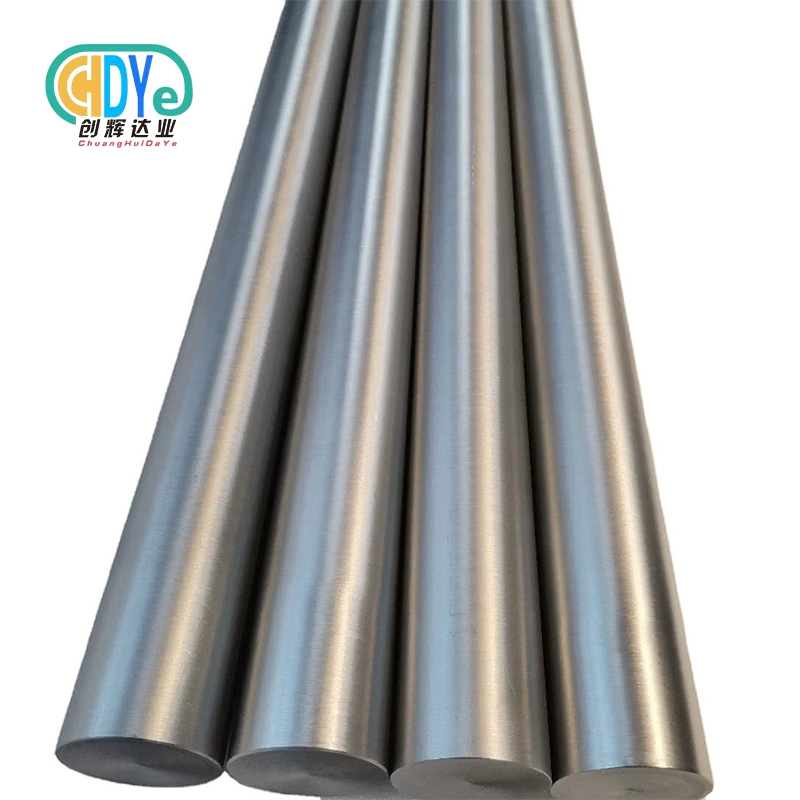
Finding reliable suppliers for medical titanium bar requires careful consideration of quality standards, certifications, and manufacturing capabilities. Medical device manufacturers, orthopedic implant producers, and surgical instrument companies worldwide depend on high-purity titanium bars that meet ASTM F67 and ASTM F136 standards. The best sources combine advanced manufacturing facilities, ISO 13485 certification, comprehensive quality control, and proven track records in biomedical applications. Whether you need Gr1, Gr2, Gr5, or Gr5 ELI grades, selecting the right supplier ensures product reliability, regulatory compliance, and successful long-term partnerships for critical medical applications.

Understanding Medical Grade Titanium Bar Applications and Specifications
Critical Applications in Modern Healthcare
Medical titanium bars serve as foundational materials over assorted healthcare applications. Orthopedic specialists depend on titanium bar orthopedic components for spinal combination bars, break obsession plates, and joint substitution stems. The extraordinary biocompatibility guarantees consistent integration with human bone tissue without activating antagonistic reactions.
Dental experts utilize titanium bar dental materials for embed projections and custom prosthetic systems. The material's osseointegration properties make lasting bonds with jawbone structures, giving steady establishments for substitution teeth. Cardiovascular applications incorporate stent fabricating and pacemaker components, where erosion safe titanium bar properties avoid corruption in body liquid environments.
Material Grades and Technical Standards
ASTM F67 covers commercially immaculate titanium grades (Gr1, Gr2) advertising predominant erosion resistance and biocompatibility for essential embed applications. ASTM F136 addresses Ti-6Al-4V ELI (Additional Moo Interstitial) amalgam, giving improved quality characteristics fundamental for load-bearing orthopedic implants.
Grade 1 titanium conveys greatest ductility and formability, making it perfect for thin-walled cardiovascular gadgets. Review 2 gives adjusted quality and erosion resistance for common surgical rebellious. Titanium amalgam bar restorative Review 5 ELI offers the most noteworthy strength-to-weight proportion, idealize for spinal bars and hip substitution stems requiring extraordinary mechanical performance.
Global Medical Titanium Bar Market Analysis
Regional Supply Chain Dynamics
China's Baoji locale, known as the "Titanium Capital," houses various specialized producers creating medical-grade titanium items. These offices advantage from vicinity to crude fabric sources and set up mechanical framework. North American providers center on high-end applications requiring exacting administrative compliance and progressed preparing capabilities.
European producers emphasize exactness machining and custom manufacture administrations for specialized restorative gadget applications. Their qualities incorporate quick prototyping capabilities and small-batch generation adaptability. Japanese providers exceed expectations in ultra-high immaculateness grades and progressed surface wrapping up strategies basic for delicate embed applications.
Demand Patterns Across Medical Sectors
Orthopedic gadget producers speak to the biggest buyer portion, requiring considerable volumes of surgical titanium bar materials for joint substitutions and injury obsession gadgets. Maturing populaces in created nations drive steady request development for hip and knee substitution components.
The dental embed segment appears quick development, especially in developing markets where healthcare get to progresses. Custom titanium bar necessities increment as personalized pharmaceutical progresses, requesting providers able of creating patient-specific geometries and dimensions.
Comparing Titanium Bar Sourcing Channels Worldwide
Direct Manufacturer Partnerships
Working straightforwardly with titanium producers offers a few focal points counting competitive estimating, customization capabilities, and quality control straightforwardness. Set up producers like Shaanxi Chuanghui Daye Metal Fabric give comprehensive administrations from crude fabric handling through last review and certification.
Direct connections empower way better communication with respect to determinations, conveyance plans, and specialized bolster. Producers prepared with vacuum bend remelting and electron bar softening offices guarantee ideal fabric immaculateness and homogeneity basic for therapeutic applications.
| Sourcing Channel | Advantages | Disadvantages | Best For |
|---|---|---|---|
| Direct Manufacturers | Competitive pricing, customization, quality control | Higher MOQs, longer lead times | Large volume orders, custom specifications |
| Local Distributors | Quick delivery, smaller quantities, local support | Higher costs, limited customization | Urgent requirements, standard grades |
| B2B Platforms | Multiple supplier options, price comparison | Quality verification challenges, communication barriers | Initial supplier research, standard products |
| Trading Companies | Consolidated sourcing, inventory management | Limited technical expertise, quality inconsistency | Multiple material requirements, inventory flexibility |
Regional Distribution Networks
Local merchants give profitable administrations counting stock administration, specialized bolster, and fast conveyance for critical necessities of medical titanium bar. They keep up connections with different producers, advertising adaptability in review choice and conveyance scheduling.
However, conveyance markups ordinarily increment fabric costs compared to coordinate producer estimating. Quality traceability may ended up complex when materials pass through different mediators some time recently coming to conclusion users.
Supplier Qualification and Verification Guidelines
Essential Quality Certifications
Medical gadget controls require providers to keep up ISO 13485 quality administration frameworks particularly planned for therapeutic gadget fabricating. This certification guarantees comprehensive quality control methods all through generation processes.
ISO 9001:2015 certification gives pattern quality administration capabilities, whereas FDA enlistment empowers providers to serve the profitable North American restorative gadget advertise. European CE stamping compliance permits advertise get to over EU part countries.
Technical Assessment Criteria
Evaluate provider fabricating capabilities counting softening forms, producing hardware, and exactness machining frameworks. Progressed offices utilize vacuum bend remelting (VAR) and electron bar softening (EBM) to accomplish the immaculateness levels basic for biocompatible titanium bar applications.
Review assessment and testing capabilities counting ultrasonic testing, chemical composition examination, and mechanical property confirmation. Providers ought to give comprehensive fabric certificates archiving chemical composition, mechanical properties, and microstructure characteristics.
Procurement Best Practices and Risk Management
Minimum Order Quantities and Pricing Strategies
Medical titanium bar MOQs change essentially based on review, measurements, and provider capabilities. Standard grades, such as Review 2 or Review 5, ordinarily require least orders extending from 100 to 500 kilograms, depending on the provider and generation handle. Custom details, counting unordinary distances across, lengths, or combination compositions, may request 1000 kilograms or higher amounts to legitimize the setup of specialized handling gear and quality confirmation conventions. The complexity of machining, warm treatment, and surface wrapping up for therapeutic applications specifically impacts the least arrange prerequisites, as producers must adjust generation effectiveness with rigid compliance standards.
Volume estimating structures regularly give considerable taken a toll diminishments at particular amount edges. Obtaining in bigger bunches can lower unit costs by spreading settled handling costs over more fabric, whereas moreover making strides conveyance effectiveness. Long-term supply understandings empower way better estimating soundness, securing buyers from advertise variances, and guarantee steady fabric accessibility for generation planning. These courses of action too bolster traceability and documentation necessities basic for administrative compliance in restorative gadget fabricating. By carefully considering MOQs, volume estimating, and supply understandings, restorative gadget producers can optimize both taken a toll proficiency and generation reliability.
Quality Control and Incoming Inspection
Establish comprehensive incoming inspection procedures including dimensional verification, surface quality assessment, and material certificate review to ensure that all medical titanium bars meet required specifications prior to production. Each lot should be examined for compliance with tolerances, surface finish standards, and documented material properties. Random sampling for independent chemical analysis and mechanical testing provides additional quality assurance beyond supplier certifications, verifying that alloy composition, hardness, tensile strength, and elongation values conform to specified medical-grade requirements. These measures help detect potential deviations early, reducing the risk of defective material entering the manufacturing process and safeguarding device performance and patient safety.
Implement traceability systems linking material lots to specific production batches and final device serial numbers. Each incoming shipment should be tagged and recorded in a centralized database to maintain full visibility of material flow. This traceability enables rapid response to quality issues, supports root cause analysis, and facilitates corrective actions when necessary. Additionally, detailed documentation of inspection results and material provenance supports regulatory compliance requirements throughout product lifecycles, including FDA, ISO 13485, and other international medical device standards. Comprehensive inspection and traceability practices ultimately enhance manufacturing reliability, ensure consistent product quality, and maintain confidence in medical device safety.
Conclusion
Successful medical titanium bar procurement requires balancing quality requirements, cost considerations, and supply chain reliability. Direct manufacturer relationships often provide the best combination of competitive pricing, customization capabilities, and quality assurance for high-volume applications. Regional distributors serve urgent requirements effectively but at premium pricing levels. Thorough supplier qualification including certification verification, facility audits, and technical capability assessment ensures long-term partnership success. Quality control procedures and comprehensive traceability systems protect against regulatory compliance issues while maintaining product integrity throughout medical device manufacturing processes.
FAQ
Q: What are the key differences between ASTM F67 and ASTM F136 titanium grades?
A: ASTM F67 covers commercially pure titanium grades (Gr1, Gr2) offering excellent corrosion resistance and biocompatibility for basic medical applications. ASTM F136 specifies Ti-6Al-4V ELI alloy providing higher strength characteristics essential for load-bearing orthopedic implants requiring superior mechanical performance.
Q: How can I verify the quality and authenticity of medical titanium bar suppliers?
A: Verify ISO 13485 and ISO 9001 certifications, request facility audit reports, review material certificates for previous orders, and conduct independent chemical analysis of sample materials. Check customer references and evaluate technical support capabilities through direct communication with engineering teams.
Q: What are typical lead times for custom medical titanium bar orders?
A: Standard grades typically require 5-7 days for production and delivery, while custom specifications may extend to 2-3 weeks depending on complexity. Rush orders for standard dimensions can sometimes be accommodated within 2-3 weeks at premium pricing levels.
Partner with Chuanghui Daye for Premium Medical Titanium Bar Solutions
Shaanxi Chuanghui Daye Metal Material delivers exceptional medical titanium bar quality backed by ISO 9001:2015 certification and three decades of rare metal industry expertise. Our Baoji facility combines advanced melting technologies with precision machining capabilities to produce ASTM F67 and F136 compliant materials. Whether you require standard grades or custom specifications, our technical team provides comprehensive support from initial consultation through final delivery. Ready to discuss your medical titanium bar supplier requirements? Contact us at info@chdymetal.com and discover why leading medical device manufacturers trust our reliable supply solutions.
References
1. American Society for Testing and Materials. "ASTM F67-13: Standard Specification for Unalloyed Titanium for Surgical Implant Applications." ASTM International, 2013.
2. American Society for Testing and Materials. "ASTM F136-13: Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI Alloy for Surgical Implant Applications." ASTM International, 2013.
3. International Organization for Standardization. "ISO 13485:2016 Medical devices - Quality management systems - Requirements for regulatory purposes." ISO, 2016.
4. Niinomi, Mitsuo. "Mechanical Biocompatibilities of Titanium Alloys for Biomedical Applications." Journal of the Mechanical Behavior of Biomedical Materials, Vol. 1, 2008.
5. Rack, Henry J., and Qudrat, Javad I. "Titanium alloys for biomedical applications." Materials Science and Engineering C, Vol. 26, 2006.
6. Williams, David F. "Titanium for Medical Applications: Principles and Applications in Clean Technology." Springer Science & Business Media, 2001.