- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Where to buy certified medical titanium plate for surgical implants?
For surgical implants, the grade and approval of the materials used are very important. Medical titanium plates are very good at being compatible with the body, strong, and resistant to rust, so they are used in many different kinds of surgery. But getting these important parts takes thought and knowledge of the business. The point of this blog post is to show healthcare workers, medical device makers, and sourcing experts where they can get approved medical titanium plates for surgical implants. We will look at sources you can trust, ways to make sure you have quality, and ways to get things quickly and reliably. When you know the important things about buying medical-grade titanium plates, you can make sure that surgical implants are safe and effective and follow strict rules.
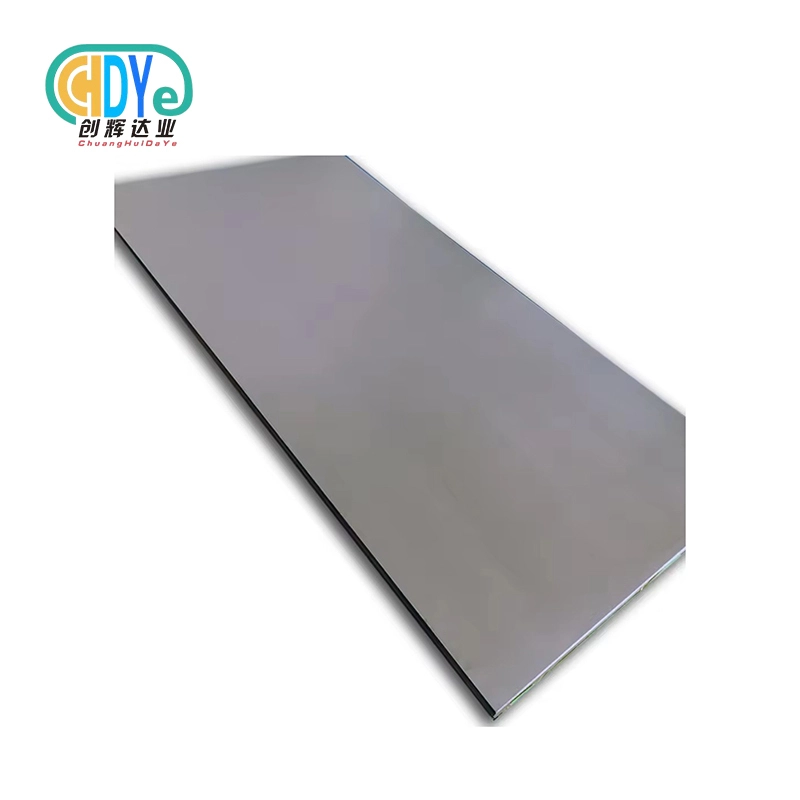
Certified suppliers of medical titanium plate for surgical implants
Reputable manufacturers with ISO certifications
It's important to work with well-known companies that have ISO certifications when you are looking for approved medical titanium plates for surgical implants. These approvals, especially ISO 13485 for medical equipment, show that a business is dedicated to managing quality and following the rules. Established makers often make medical-grade titanium plates, which ensures that the quality and dependability are always the same. Grade 5 (Ti-6Al-4V) and Grade 23 (Ti-6Al-4V ELI) titanium alloys are often used in medical settings and are usually part of the large selection offered by the company. These providers usually help you with your particular implant needs by giving you technical support, thorough material certifications, and traceability documents.
Specialized medical device distributors
Specialized medical gadget dealers are another good place to get certified medical titanium plates. Manufacturers and end-users deal with these companies, which often have a wide range of medical-grade goods in stock. There are many benefits to working with a well-known wholesaler. They can give you access to multiple brands, lower prices, and quicker delivery times. A lot of wholesalers can do extra things like surface treatments, special cutting, or packaging, and they also have their own ways of checking quality. When choosing a wholesaler, make sure they are certified, well-known in the industry, and able to provide full paperwork for the medical titanium plates they sell.
Direct-to-manufacturer online platforms
In the last few years, direct-to-manufacturer online sites have become a handy way to get approved medical titanium plates. These platforms connect buyers directly with verified makers. This often makes the buying process easier and saves money. A lot of these websites have features like quick quoting, material comparisons, and making it easy to get technical specs and licenses. When using these platforms, you should carefully check the makers on the list to make sure they meet all quality and legal standards for medical-grade titanium plates. Find sites that focus on medical supplies and have strong ways of checking the sellers they list.
How to verify medical titanium plate quality and compliance
Understanding material certifications and standards
A deep knowledge of material approvals and standards is the first step in making sure that medical titanium plates are safe and effective. ASTM F67 and ASTM F136 are important rules that say how titanium must be chemically made and how it must work mechanically, along with other things, when it is used in medical implants. You should always ask for and read material test results (MTRs) or certificates of analysis (COAs) when you buy medical titanium plates. These papers should explain the chemical makeup, mechanical qualities, and any extra tests that were done on that certain group of titanium plates. Know the acceptable limits for each element and trait so that you can be sure the material meets the needs for surgical implants.
Third-party testing and validation
To be sure that medical titanium plates are made correctly and meet all standards, you could have someone else try and confirm them. A lot of different tests can be done by independent labs, such as looking at chemicals, trying mechanical properties, looking at microstructures, and checking biocompatibility. These fair tests show that the manufacturer's certificates aren't the only proof of quality. When you choose a third-party testing center, look for one that is certified to ISO 17025 standards. This shows that they know how to do certain tests. Getting the plates tested by a third party regularly can help you find any problems with the quality of the materials and feel good about using the titanium plates in surgical implants.
On-site audits and supplier qualifications
For companies that need a lot of medical titanium plates or have certain quality needs, checking possible sources in person can be very helpful. These checks let you look at the manufacturer's way of making things, their quality control methods, and how well they follow industry rules. Check the supplier's testing, production tools, raw material handling, and paperwork during a check. You should also look at how they handle quality, train employees, and follow the rules. A strong seller qualification process with frequent re-audits helps make sure that the medical titanium plates used in your surgical implants are always of the same quality and follow the rules.
Where to source medical titanium plate quickly and reliably
Establishing long-term supplier partnerships
One of the best ways to make sure that medical titanium plates can be quickly and reliably sourced is to build long-term relationships with sellers you trust. By building good connections with makers or distributors, you can often get faster response times, committed inventory, and priority production slots. Better communication and teamwork are possible with these relationships. This lets suppliers better understand your needs and may even lead them to customize their products for you. When choosing a long-term partner, look at how stable their finances are, how much they can produce, and how committed they are to finding new ways to use medical-grade titanium products.
Utilizing just-in-time inventory systems
Using a just-in-time (JIT) stocking system can help you get medical titanium plates much faster and more reliably. This method means working closely with sellers to get goods when they are needed. This way, you have what you need without having too much extra stock. If you want to successfully adopt a JIT system for medical titanium plates, you should work with your providers to make sure you can talk clearly, track inventory in real time, and streamline the buying process. This approach not only makes it easier to find supplies, but it also lowers carrying costs and the risk of material failure. This is especially important for medical-grade titanium because it is very valuable and has specific needs.
Leveraging global supply networks
In today's world, which is linked, using global supply networks can help you get approved medical titanium plates quickly and reliably. If you get supplies from a range of different areas, you can lower the risks that come with problems in those areas and make the most of different production abilities and wait times. When you make your supply network global, think about things like how you'll move goods, the rules for importing and exporting, and possible language or culture barriers. It's also very important to make sure that all of the sellers from other countries meet the same strict quality and safety standards that are needed for medical-grade titanium plates. Building ties with logistics companies that are good at handling medical products can help you get even more reliable and faster with your global sourcing.
Conclusion
Sourcing certified medical titanium plates for surgical implants requires careful consideration of supplier credentials, quality verification methods, and efficient procurement strategies. By partnering with reputable manufacturers, utilizing specialized distributors, and leveraging online platforms, healthcare professionals and medical device manufacturers can ensure access to high-quality materials. Implementing robust quality control measures, including third-party testing and supplier audits, is crucial for maintaining compliance and patient safety. Establishing long-term supplier relationships, adopting JIT inventory systems, and tapping into global supply networks can significantly enhance the speed and reliability of sourcing efforts. As the demand for medical titanium plates continues to grow, staying informed about industry standards and best practices is essential for success in this critical field.
For those seeking a reliable supplier of medical titanium plates and other high-quality metal materials, Shaanxi Chuanghui Daye Metal Material Co., Ltd. offers a comprehensive range of products and services. Located in China's "Titanium Capital," the company combines decades of industry expertise with advanced manufacturing capabilities to deliver superior titanium products for medical applications. Their commitment to quality, customer satisfaction, and regulatory compliance makes them an excellent partner for sourcing certified medical titanium plates. For more information or to discuss your specific requirements, please contact them at info@chdymetal.com.
FAQ
Q: What are the most common grades of titanium used for medical implants?
A: The most common grades are Grade 5 (Ti-6Al-4V) and Grade 23 (Ti-6Al-4V ELI), known for their excellent biocompatibility and mechanical properties.
Q: How often should I audit my medical titanium plate suppliers?
A: It's recommended to conduct initial audits before partnering and then perform follow-up audits every 1-2 years, or more frequently if there are quality concerns.
Q: What key certifications should I look for when choosing a medical titanium plate supplier?
A: Look for ISO 13485 certification for medical devices, as well as compliance with ASTM F67 and ASTM F136 standards for titanium materials.
Q: Can I use online platforms to source medical-grade titanium plates?
A: Yes, but ensure the platform specializes in medical materials and thoroughly vet the manufacturers listed for quality and compliance.
Q: What documentation should I expect when purchasing certified medical titanium plates?
A: You should receive material test reports (MTRs) or certificates of analysis (COAs) detailing chemical composition, mechanical properties, and any additional testing performed.
References
1. ASTM International. (2020). ASTM F67-13(2020) Standard Specification for Unalloyed Titanium, for Surgical Implant Applications.
2. ASTM International. (2021). ASTM F136-21 Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant Applications.
3. International Organization for Standardization. (2016). ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes.
4. Rack, H. J., & Qazi, J. I. (2006). Titanium alloys for biomedical applications. Materials Science and Engineering: C, 26(8), 1269-1277.
5. Sidambe, A. T. (2014). Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials, 7(12), 8168-8188.
6. Wang, K. (1996). The use of titanium for medical applications in the USA. Materials Science and Engineering: A, 213(1-2), 134-137.
Learn about our latest products and discounts through SMS or email