- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
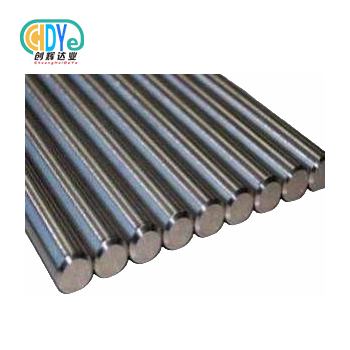
What Grade Medical Titanium Bar Is Best for Surgical Use?
Review 5 ELI (Additional Moo Interstitial) titanium bars are best for imperative surgical applications. These restorative medical titanium bar items are biocompatible and solid, with a malleable quality of 860 MPa and erosion resistance in physiological liquids. The ELI assignment diminishes oxygen and nitrogen, moving forward tissue compatibility and embed execution for orthopedic, dental, and cardiovascular applications.
Understanding Medical Titanium Bars and Their Grades
Titanium bars underpin surgical implants and precise tools. These unique metal rods must meet strict purity, mechanical, and biocompatibility standards for patient safety and implant lifespan. Procurement experts must understand titanium grades since they affect surgical outcomes.
Common Titanium Grades in Medical Applications
Four titanium grades are utilized in medicine, each with special properties. Review 1 titanium is perfect for dental crowns and surgical work due to its tall erosion resistance and malleable quality. Review 2 titanium is utilized in orthodontic and cardiovascular stents for its erosion resistance and mechanical strength.
Grade 5 titanium (Ti-6Al-4V) is suitable for load-bearing orthopedic inserts counting hip and knee substitutions, due to its 895 MPa pliable quality. The updated Review 5 ELI has fewer interstitial components to increase biocompatibility and mechanical execution for key surgical applications.

Manufacturing Excellence and Quality Standards
Each therapeutic titanium bar we make is immaculate and homogeneous thanks to predominant vacuum arc remelting (VAR) and electron bar softening (EBM) innovation. Hot manufacturing, rotating swaging, and accurate rolling give steady grain structures that progress weakness resistance and mechanical durability.
The controlled tempering handle improves mechanical and weakness qualities, whereas centerless pounding, fixing, and cleaning reach ±0.01 mm dimensional tolerances. ASTM F136 and ASTM F67 benchmarks are taken after all through manufacturing to comply with worldwide restorative device laws and guarantee traceability.
Key Properties of Medical Titanium Bars for Surgical Use
Titanium bars' biocompatibility, mechanical strength, and corrosion resistance make them ideal for surgery. Titanium is favored over stainless steel and cobalt chrome alloys due to its characteristics.
Biocompatibility and Tissue Integration
Biocompatibility is the most vital aspect of surgical titanium implants. Tissue compatibility without cytotoxicity or unfavorably susceptible reaction permits bone embed osseointegration with restorative titanium bars. Stretch-protecting impacts that cause bone resorption around inserts are diminished by the material's highly versatile modulus, which matches human bone.
The common oxide layer of titanium ensures against physiological liquids and particle release that might cause aggravation. Clinical thinks about appear titanium inserts appear to have long-term integration rates over 95%, demonstrating their unwavering quality for lasting surgery.
Mechanical Performance Characteristics
Mechanical characteristics vary widely across grades of medical titanium bars, affecting their surgical appropriateness. Understanding these traits helps choose materials for implant needs and load-bearing circumstances.
Grade 5 ELI titanium bars have a tensile strength of > 860 MPa and a yield strength of ≥ 795 MPa, making them ideal for orthopedic applications. Elongation of ≥ 10% and area reduction of ≥ 25% ensure ductility for complex implant geometries and fatigue resistance under cyclic loading.
The density of 4.43 g/cm³ provides weight advantages over stainless steel, lowering implant mass without losing strength. In spinal implants and major orthopedic prostheses, reducing patient load improves recovery.
Corrosion Resistance and Sterilization Compatibility
Medical titanium bars resist bodily fluids, saline solutions, and surgical sterilization. Electrochemical corrosion is prevented throughout implant life by the passive oxide layer's quick repair.
Steam, gamma, and ethylene oxide sterilization do not damage titanium's mechanical or surface qualities, maintaining implant integrity during processing and surgery. Medical device production and hospital sterilization are simplified by this compatibility.
How to Choose the Best Grade Medical Titanium Bar for Surgical Applications
Surgical, mechanical, and regulatory needs must be considered while choosing a titanium grade. Performance, cost, and production feasibility must be considered.
Application-Specific Grade Selection Criteria
Hip stems, knee components, and spinal rods need Grade 5 or Grade 5 ELI titanium bars to sustain physiological loads and cyclic loading. High tensile and yield strengths ensure implant longevity in biomechanically demanding situations.
Grade 5 ELI titanium bars improve biocompatibility and osseointegration for dental implants. Interstitial fluid reduction reduces inflammation while retaining mechanical strength for masticatory loads.
Grade 2 titanium bars are used in cardiovascular and soft tissue applications when biocompatibility trumps mechanical strength. Pacemaker housings, surgical clips, and vascular repair devices require corrosion resistance and tissue compatibility.
Procurement Considerations and Supplier Evaluation
When buying surgical titanium bars, quality certification is essential. Suppliers must meet ISO 13485, ISO 9001, ASTM F136, and ASTM F67 requirements for product reliability and regulatory compliance.
Assessment of manufacturing competence should include melting, forming, heat treatment, and precision machining. Advanced inspection methods include ultrasonic testing, chemical composition analysis, and mechanical property verification guarantee product quality and traceability.
Suppliers can customize dimensions, surface treatments, and mechanical property optimization for surgical needs. Flexible manufacturing supports prototype development, small-batch production, and large-volume supply contracts for different procurement needs.
Comparative Analysis: Grade 5 vs Grade 5 ELI Performance
Both grades have amazing mechanical qualities, but Review 5 ELI has less oxygen, nitrogen, and carbon, making strides in biocompatibility. In spite of the fact that fabric costs are higher, this alteration moves forward long-term embed viability and diminishes complications.
Grade 5 titanium is cheaper for outside obsession gadgets and surgical instruments where biocompatibility is not imperative. The ordinary review works well for brief inserts and non-permanent surgery.
Grade 5 ELI is best for long-term tissue integration in changeless inserts, such as joint replacements, spinal fusion devices, and dental embed frameworks. Made strides, persistent results, and lower amendment surgery rates legitimize the higher virtue costs.
Procurement Guide for Medical Titanium Bars: Where and How to Buy
Medical titanium bars must be sourced from vendors with excellent production, quality assurance, and global distribution networks. To achieve project success, procurement must address technical specifications, regulatory compliance, and supply chain reliability.
Supplier Selection and Certification Requirements
Qualified providers must have an ISO 13485 therapeutic device and an ISO 9001 common quality administration framework. This certification guarantees efficient quality control, hazard administration, and nonstop enhancement all through manufacturing.
Suppliers must give fabric certificates, test reports, and traceability printed material for each generation parcel for ASTM F136 and F67 compliance confirmation. Restorative gadget compliance is demonstrated by ICP-OES chemical composition investigation, mechanical testing, and biocompatibility verification.
Manufacturing plant reviews evaluate efficiency, quality control, and defect avoidance. Progressed dissolving gear, accuracy machining, and careful reviews illustrate provider's status to meet restorative gadget criteria.
Custom Manufacturing and Specification Development
Custom titanium bar specs are commonly needed for medical devices. Flexible manufacturers can meet demanding surgical application diameter, length, and tolerance requirements.
Implant designs and manufacturing processes can be optimized using surface finish modification. Surface roughness below Ra 0.2 μm in polished, pickled, or mirror finishes fulfills machining needs and enhances implant performance.
Controlled heat treatment optimizes strength, ductility, and fatigue resistance for specific loading circumstances. Innovative implant designs and surgical techniques benefit from this customization.
Logistics and Delivery Considerations
International transportation of medical titanium bars requires particular packaging to prevent contamination and damage. Clean packaging, protective materials, and secure containers preserve product integrity from manufacturing to the consumer.
For project timelines, lead time planning must account for material manufacture, quality testing, and shipment. Standard specs take 4-8 weeks, but custom and large-volume orders take longer.
Consignment programs, blanket purchase orders, and just-in-time delivery maximize working capital and ensure production schedules. Flexible methods facilitate prototype development and large-scale manufacture.
Chuanghui Daye: Your Trusted Medical Titanium Bar Manufacturer
Shaanxi Chuanghui Daye Metal Material Co., Ltd., China's "Titanium Capital," manufactures medical devices using rare metals with over 30 years of experience. Our company manufactures high-grade surgical titanium bars with ISO 9001:2015 certification and strict quality control.
Advanced Manufacturing Capabilities and Quality Excellence
Our production facility features state-of-the-art melting, forging, rolling, and precision machining equipment dedicated to medical-grade titanium materials. Vacuum arc remelting (VAR) and electron beam melting (EBM) processes ensure exceptional purity and homogeneity in every medical titanium bar we produce.
Comprehensive inspection procedures validate product quality at every manufacturing stage. These quality measures demonstrate our commitment to excellence and regulatory compliance:
• 100% ultrasonic testing for internal defect detection ensures structural integrity throughout each bar's length
• Chemical composition analysis using ICP-OES technology verifies compliance with ASTM F136 and ASTM F67 specifications
• Mechanical testing, including tensile, hardness, and impact evaluation, confirms performance characteristics
• Microstructure examination ensures uniform α+β phase distribution for optimal mechanical properties
• Biocompatibility and cytotoxicity testing validate implant compliance and patient safety requirements
Our advanced quality control systems provide complete traceability from raw material sourcing through final delivery, ensuring product reliability for medical implant manufacturers worldwide.
Product Specifications and Customization Services
We make medical titanium bars in Grades 1, 2, 5, and 5 ELI with 4mm–20mm diameters and 2500mm–3000mm lengths. H7, h8, and H9 precision tolerances meet different machining and implant standards.
Surface treatments, including polished, pickled, and mirror, attain roughness values below Ra 0.2 μm, enabling surgical and manufacturing uses. Orthopedic, dental, and cardiovascular applications with unique performance requirements can use custom dimensions and alloy compositions.
Our technical team works with customers to optimize surgical solutions and ensure material qualities meet implant design objectives and regulatory compliance criteria.
Conclusion
The right medical titanium bar grades affect surgical implant function, patient safety, and long-term clinical outcomes. Grade 5 ELI titanium bars are best for important surgical applications due to their biocompatibility and mechanical strength. Understanding titanium grade qualities, manufacturing processes, and quality criteria helps procurement experts make cost-effective and performance-optimal decisions. Certified manufacturers with superior production capabilities, complete quality assurance, and customization flexibility provide reliable titanium products that meet medical device regulations and promote effective surgical outcomes.
FAQ
Q: What are the key differences between Grade 5 and Grade 5 ELI titanium bars?
A: Grade 5 ELI (Extra Low Interstitial) titanium contains reduced levels of oxygen, nitrogen, and carbon compared to standard Grade 5 titanium. This reduction enhances biocompatibility and tissue integration while maintaining similar mechanical strength properties. Grade 5 ELI is specifically designed for permanent implant applications requiring long-term tissue compatibility.
Q: How does titanium compare to stainless steel for surgical implants?
A: Titanium offers superior biocompatibility, corrosion resistance, and lower elastic modulus compared to stainless steel. The density of titanium (4.43 g/cm³) is approximately 45% lower than that of stainless steel, reducing implant weight. Titanium's natural oxide layer provides better protection against bodily fluids, while its elastic modulus more closely matches bone properties.
Q: Can medical titanium bars be customized for specific implant designs?
A: Yes, reputable manufacturers offer customization services including diameter modifications, length adjustments, surface finish optimization, and mechanical property tailoring. Custom specifications can accommodate unique surgical requirements, prototype development, and specialized implant geometries while maintaining regulatory compliance.
Q: What quality certifications should suppliers provide for medical titanium bars?
A: Suppliers must demonstrate ISO 13485 and ISO 9001 certification alongside compliance with ASTM F136 and ASTM F67 standards. Material certificates, chemical composition analysis, mechanical testing results, and traceability documentation should accompany each shipment to ensure regulatory compliance and product reliability.
Partner with Chuanghui Daye for Premium Medical Titanium Bar Solutions
Choosing the right medical titanium bar supplier directly impacts your surgical device quality and patient outcomes. Shaanxi Chuanghui Daye combines three decades of rare metal expertise with advanced manufacturing capabilities to deliver exceptional titanium solutions for medical device manufacturers. Our ISO 9001:2015 certified facility produces medical titanium bars meeting ASTM F136 and ASTM F67 standards, ensuring consistent quality and full traceability for your critical applications. Contact our technical team at info@chdymetal.com to discuss your specific requirements and receive detailed quotations for your medical titanium bar procurement needs.
References
1. American Society for Testing and Materials. Standard Specification for Unalloyed Titanium, for Surgical Implant Applications. ASTM International, 2017.
2. Williams, David F. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications. Springer Science & Business Media, 2001.
3. Rack, Henry J., and Quesada, Jaime V. Titanium alloys for biomedical applications. Materials Science and Engineering, 2006.
4. Long, Marc, and Rack, Henry J. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials Research Advances, 1998.
5. Brunette, Daniel M. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications. Engineering Materials, 2001.
6. Geetha, Manivasagam, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants – A review. Progress in Materials Science, 2009.
Learn about our latest products and discounts through SMS or email
_1760923994993.jpg)